-
Flap’s Blog.com Links and Comments for November 4th
These are my links for November 4th.
- Stroke Damage to Insular Cortex Boosts Smoking Cessation– Smokers who suffer a stroke that causes a lesion at the insular cortex are more than 5 times more likely to stop their nicotine habit than those whose stroke did not result in such a lesion, according to a new study.In addition, the researchers found that preparedness to change also influenced successful smoking cessation poststroke.
The study results were not surprising, given that research has already shown that biological and psychological factors help explain smoking cessation in patients with stroke, said the study’s lead author, Rosa Su?er Soler, PhD, from the Neurology Department, Josep Trueta Hospital, Girona, Spain.
Biologically, the insular cortex may play an important role in emotional decision-making, and in terms of psychology, smoking behavior may be explained by stages, processes, and levels of change, Dr. Su?er told Medscape Medical News. “Before you stop smoking, you must be aware that you have a problem and take the decision to stop smoking.”
The study was published online November 3 in Stroke.
- Vaccination Exemptions Rise in California Amid Concerns– Increasing rates of unvaccinated young children with “personal belief exemptions” from vaccination requirements are becoming worrisome, according to research presented here at the American Public Health Association (APHA) 139th Annual Meeting.Recent concern about vaccine safety appears to be gaining strength, and state regulations requiring parents to vaccinate their children before they can attend public schools vary. In California, obtaining a personal belief exemption could not be easier — parents are only required to sign their name to a 2-sentence standard exemption statement on the back of the vaccination requirement form.
In evaluating data on the rates of exemptions from the California Department of Public Health, the state’s Department of Education and the US Census, researchers found that in 2010, the state had about 11,500 kindergartners with personal belief exemptions, representing a 25% increase over the previous 2 years.
The increasing rate indicates that, for kindergartners who have adhered to vaccination schedules, exposure to children with personal belief exemptions is about 2.3 per 100 children.
Because children with the exemptions tend to be found in clusters, the rate of children with exemptions who are exposed to other children who also have exemptions — a higher-risk combination — was 15.6 per 100 in 2010, said lead author Alison Buttenheim, PhD, MBA, from the University of Pennsylvania’s Robert Wood Johnson Health & Society Scholars Program in Philadelphia.
“The average kindergartner with a personal belief exemption attends a school where the exemption rate is 15 per 100, and we see that figure increasing all the time,” she reported.
Previous data from the fall of 2008 showed that 10% of the nearly half-million kindergartners in California attended schools where personal belief exemption rates exceeded 5%, and as many as 61% of kindergartners with 1 or more personal belief exemptions (n = 9196) attended schools where the personal exemption rate exceeded 5%. Among those, a third attended schools where the personal belief exemption rate exceeded 20%.
In a separate study conducted by the same team, the researchers investigated the concerns that parents have about vaccines by evaluating data on the specific vaccines received by 168 patients at a pediatric practice in Philadelphia where the practitioner, though pro-vaccine, is known to accommodate parents who seek alternative vaccination options.
- Sales Taxes and the Internet– Online commerce is a big, big business, accounting for nearly one-tenth of retail sales in the United States. It is a lively and growing sector, a bright spot in our troubled economy — thus the gloomy shadow of the taxman inevitably falls upon it, in the form of a bill proposed by Republican senators Mike Enzi of Wyoming and Lamar Alexander of Tennessee. A similar bill was proposed by Democratic senator Dick Durbin of Illinois earlier in the year, and a separate effort is afoot to have the so-called supercommittee institute new Internet-tax measures as part of its deficit-reduction plan.But it’s not all about big business: The Enzi-Alexander bill would affect entrepreneurs with as little as $500,000 a year in sales.
Contrary to most accounts, there is no sales-tax loophole for online retailers. Customers who buy goods online are in most cases required to pay a “use tax” equivalent to the sales tax they would have paid in a conventional transaction. The problem, from the tax-consumers’ point of view, is that most taxpayers do not comply with the law. The state and local governments that depend upon sales-tax revenue protest that they are strapped for cash. That isn’t entirely true, either: Those jurisdictions are spending more money than ever, most of it on salaries and benefits for the legion of bureaucrats and commissars they maintain.
But in spite of their swollen payrolls and work forces, state and local governments apparently cannot be bothered to hire tax agents in sufficient numbers, thus the now universal practice of their requiring businesses to do their sales-tax collecting for them. The Internet-tax measures under consideration would not expand governments’ power to tax, but its power to conscript businesses into acting as tax collectors.
The original sin here is government’s delegating its tax-collecting duties to private businesses. If government wishes to levy a tax, let it do the work of collecting it. It is true that this would prove burdensome to cities and states. It is also burdensome to the conscripted businesses. The difference is that collecting taxes is government’s duty, not Amazon’s.
- Stu’s Dangerous Dozen: Unsafe House Incumbents – Dan Lungren (R-Calif.). Another election means another problem for Lungren, who somehow wins despite his reluctance to raise money. He will be running in a 46 percent McCain district this time, compared with the 48 percent McCain district he ran in last time, but he also will draw the same opponent, Ami Bera. Bera, a doctor who raises money nationally from Indian-Americans, ran a competitive race in a terrible year for a Democrat, so he hopes the better environment will help him close the 7-point gap he had in 2010.
- GOP Candidate Beats Obama in Swing States on Jobs, Deficit – Voters in 12 key swing states are substantially more likely to feel that a generic “Republican candidate” for president would do a better job than President Obama of handling the federal deficit and debt, and are slightly more likely to prefer the Republican on the issue of unemployment. Swing-state voters are split on the question of whether Obama or the Republican candidate would do a better job of handing healthcare as well as terrorism and international threats.
- Colgate recalls mouthwash over contamination fears– Colgate-Palmolive is removing up to 50,000 bottles of Periogard mouthwash from store shelves in the U.K. due to possible bacterial contamination.The micro-organisms may be harmful to some people with weakened immune systems or some lung conditions, according to the company.
Up to 11 other countries, including some where the product has a different brand name, are also involved in the recall of 300-mL containers containing chlorhexidine.
“The presence of micro-organisms has been detected in some retained production samples of Periogard,” Colgate-Palmolive said in a statement. “Under certain circumstances, these micro-organisms may be harmful to individuals with compromised health. Accordingly, in order to ensure the safety of our consumers, in cooperation with the Medicine and Health Regulatory Authority, Colgate-Palmolive UK is recalling all Periogard.”
- ADA updates guidelines for managing ONJ risk patients– A patient receiving antiresorptive therapy for the prevention and treatment of osteoporosis has a low risk of developing osteonecrosis of the jaw (ONJ), and benefits of the medication outweigh the risk of ONJ, according to an advisory statement from the ADA.The statement, “Managing the Care of Patients Receiving Antiresorptive Therapy for Prevention and Treatment of Osteoporosis,” is based on a literature review by an advisory committee of the ADA Council on Scientific Affairs and updates ADA’s 2008 advisory statement (Journal of the American Dental Association, November 2011, Vol. 142:11, pp. 1243-1251).
ONJ associated with antiresorptive agents has mostly been referred to as bisphosphonate-associated ONJ, but nonbisphosphonate antiresorptive agents are now available that also could be associated with ONJ, the panel noted. That is why they refer to the condition as antiresorptive agent-induced ONJ (ARONJ).
A relatively new condition, bisphosphonate-associated ONJ, has received tremendous media attention because of a flurry of lawsuits against the makers of Fosamax and Zometa alleging that the medications led to ONJ.
These lawsuits have been a factor in raising patients’ and dentists’ awareness of the condition, according to Helen Ristic, PhD, director of scientific information for the ADA’s Division of Science and one of the panelists who contributed to the report.
- Democratic Congressional Campaign Committee Targets California GOP Representatives With Ad Campaign » Flap’s California Blog – Democratic Congressional Campaign Committee Targets California GOP Representatives With Ad Campaign
- Flap’s Dentistry Blog: Do Those Wisdom Teeth REALLY Need to Come Out? – Do Those Wisdom Teeth REALLY Need to Come Out?
- The Afternoon Flap: November 4, 2011 | Flap’s Blog – FullosseousFlap’s Dental Blog – The Afternoon Flap: November 4, 2011 #tcot #catcot
- Cain accuser stands by sexual harassment complaint – CNN.com – Cain accuser stands by sexual harassment complaint
- Stroke Damage to Insular Cortex Boosts Smoking Cessation– Smokers who suffer a stroke that causes a lesion at the insular cortex are more than 5 times more likely to stop their nicotine habit than those whose stroke did not result in such a lesion, according to a new study.In addition, the researchers found that preparedness to change also influenced successful smoking cessation poststroke.
-
FDA Panel Says Bisphosphonates Need Label Changes on Use Duration

This is the recommendation that came down today although the Food and Drug Administration does not have to accept the recommendations of its panels.Bone drugs from Warner Chilcott Plc, Roche Holding AG, Merck & Co. and Novartis AG need labeling changes to reduce the risk of fractures, a U.S. panel said.
The companies should add clarifications on the length of time that osteoporosis patients may take the medicines, outside advisers to the Food and Drug Administration said today in a 17- 6 vote in Adelphi, Maryland. The FDA isn’t required to follow its panels’ recommendations.
The agency has evaluated the safety of the drugs, known as bisphosphonates, for almost four years and cited possible links to unusual thigh fractures and jawbone deterioration in 2010. The agency said in July it also was examining conflicting studies on whether bisphosphonate pills such as Warner Chilcott’s Actonel, Merck’s Fosamax and Roche’s Boniva raise esophageal cancer risks.
A revised label should “be very clear that efficacy may fall off after a period of time, perhaps five years,” panelist Lewis Nelson, director of the medical toxicology fellowship program at New York University, said after the vote. “Serious concerns have been raised about risk, and those need to be continually evaluated as well.”
There needs to be additional research, period.
Mere warning labels are not going to answer the questions from every day patients – how long do I take the medicine and what protocol do I use? Or, what is the chance my femur will fracture or will I develop osteonecrosis of the jaw (ONJ) if I have a tooth removed.
-
Fosamax Osteonecrosis of the Jaw Lawsuit Set for Trial September 7

Osteonecrosis of the Jaw (ONJ)
Another oral bisphosphonate (Fosamax with ONJ) lawsuit trial set.The next trial date for a Fosamax lawsuit involving jaw necrosis is scheduled to begin early next month.
The Fosamax trial will involve a complaint brought by Linda Secrest, of Florida, who alleges that Merck failed to adequately warn that side effects of Fosamax, the popular osteoporosis drug, can lead to severe jaw bone decay. Trial is scheduled to begin on September 7, in the U.S. District Court for the Southern District of New York.
Fosamax is an osteoporosis drug that belongs to a family of similar medications known as bisphosphonates. Long-term use of oral bisphosphonates has been linked to an increased risk of serious and debilitating jaw problems, known as osteonecrosis of the jaw. The condition causes the jaw bone to decay and rot, often resulting in the need for surgery to remove portions of the jaw.
Merck currently faces more than 1,100 Fosamax jaw lawsuits, most of which have been consolidated and centralized for pretrial proceedings in the U.S. District Court for the Southern District of New York as part of an MDL or multidistrict litigation.
There have already been several trials for Fosamax jaw necrosis lawsuits that have been held in the federal MDL over the last two years. While Merck has successfully defended its medication in three cases, one lawsuit resulted in an $8 million jury award for Fosamax jaw damage last year, finding that Merck failed to adequately research the potential side effects of warn about the risk of jaw necrosis from Fosamax.
A few more cases and Merck will probably propose some industry-wide settlement agreement.
Next up will be the lawsuits over bone fractures from Fosamax.
-
Flap’s Links and Comments for May 6th on 08:32
These are my links for May 6th from 08:32 to 08:45:
- What Will Be the Future for Long Time Fosamax Users? – While jumping rope with the neighborhood children, a 59 year old Queens, New York woman felt her thigh bone snap. The pain was so severe that she fell to the ground as she readied for another jump. Sandy Potter had been diagnosed at the age of 48 with osteoporosis and began taking the drug Fosamax. She further stated that she had been on the medication for eight years prior to the incident and was now informed that her femur had snapped into two separate pieces.
Fosamax is a commonly described drug used in the treatment of osteoporosis and contains bisphosphonates, which is used to slow the loss of bone and increase bone mass. Concerns about some of the side effects of Fosamax have been increasing and now there is mounting evidence that for some women that have been taking Fosamax for more than five years could be in danger of having fractures that are spontaneous in nature.
According to Dr. Kenneth Egol, a professor of orthopedic surgery at NYU Langone Medical Center, over the last 18 months, femur fractures, such as the one in Sandy Potter’s case are occurring with more frequency
Many patients claim that they were engaged in a low-impact exercise when they suddenly experience a break in the femur. What concerns Dr. Egol, is that because the femur is known as one of the strongest bones in the body, it should not be sustaining the damage that it has. Yet, people taking a leisurely walk or walking down a flight of stairs is experiencing this type of injury. Dr. Egol further stated that upon reviewing the X-rays taken of some of his patients, the images take on the appearance of an injury endured by a car accident rather than a minimal fall. - Dietary changes may preclude some prescriptions for osteoporosis, researchers say – While a number of prescription medications exist to reverse the bone loss associated with osteoporosis, a recent survey of medical literature indicated that many patients should first look into getting more calcium and vitamin D in their diets or through supplementation.
Researchers from the University of Illinois at Urbana-Champaign stated as much in an article published in the journal Nutrients, where they noted that bisphosphonates may not rebuild the best sort of bone tissue, compared to adequate vitamins and nutrients in one's meals.
In a meta-analysis of more than 200 research papers published in the prior 10 years, scientists found that the direction of medical inquiry has been increasingly swinging toward the initial use of calcium and vitamin D to treat osteoporosis.
Co-author Karen Chapman-Novakofski said that a partial explanation for this trend appears to be the difference in bone quality between individuals on bisphosphonates and those eating a more complete diet or taking dietary supplements.
"Bisphosphonates…disrupt normal bone remodeling by shutting down the osteoclasts – the cells that break down old bone to make new bone," she commented. "When that happens, new bone is built on top of old bone. Yes, your bone density is higher, but the bone's not always structurally sound."
The National Osteoporosis Foundation states that bisphosphonates decrease the risk of hip fracture by up to 50 percent. However, the organization adds that some researchers have raised concerns that the drug class can occasionally lead to negative side effects, including thigh aches and broken femurs.By contrast, people over the age of 50 who take 1,200 milligrams of calcium and up to 1,000 international units of vitamin D each day may see gains in bone density with relatively few side effects, Chapman-Novakofski said. - Osteoporosis Drugs, Bisphosphonates Increases the Risk of Fractures – According to a new study at Sweden, it has been disclosed that osteoporosis drugs known as bisphosphonates is linked to the infrequent bone fractures. However, the risk of the fractures from osteoporosis drugs is very small in contrast to their benefits.
The bone-building drugs bisphosphonates includes Aclasta, Actonel, Aredia, Bondronat, Boniva, Didronel, Fosamax, Fosavance, Reclast, Skelid, and Zometa.
A Study Researcher and Professor of Orthopaedic Surgery at Linkoping University, Sweden, Per Aspenberg, MD, and PhD said that the risks of these drugs are very rare if it is prescribed correctly.
The study, which was published in the New England Journal of Medicine, has included 12,777 women aged 55 and older, who suffered a fracture of the thigh bone in 2008. The study found that osteoporosis drugs are associated with the higher risks of fractures. The association among the bone-building drugs and fractures is very weak.
- Bisphosphonate use and ?risk of atypical hip and ?femoral shaft fractures – Clinical question Do oral bisphosphonates increase the risk of atypical hip and femur fractures in postmenopausal women?
Bottom line Oral bisphosphonate use for 5 years or longer is significantly associated with an increased risk of atypical hip and femur fractures (subtrochanteric or femoral shaft). A previous study (JAMA 2006;296:2927-2938) found little if any benefit of extended bisphosphonate use beyond 5 years in reducing the risk of typical fractures of the femoral neck or intertrochanteric region. Although this study design (case-control) is classified as weak evidence (LOE = 3b), it may be the best we have for some time. Thus, practicing clinicians should consider stopping bisphosphonates for most women after 5 years, except for those at high risk (eg, chronic steroid users). (LOE = 3b)
Synopsis Current evidence on whether bisphosphonates increase the risk of subtrochanteric or femoral shaft fractures in postmenopausal women is uncertain. These investigators analyzed information obtained from multiple databases on the association between bisphosphonate use and fractures in a cohort of women in Ontario, Canada, 68 years or older, who initially started oral bisphosphonate (alendronate, risedronate, or etidronate) therapy between April 2002 and March 2008. Separate databases included information on drug prescriptions, hospitalizations, physician service claims, cancer, and basic demographic information. Cases included those women hospitalized with a subtrochanteric or femoral shaft fracture. Up to 5 women who were not hospitalized with similar fractures were matched to age and cohort entry date and served as control patients. Analyses were performed to adjust for other fracture risk factors. During the 7-year study period, 205,466 women commenced oral bisphosphonate therapy. Of these, 716 (0.35%) were hospitalized for an atypical subtrochanteric or femoral shaft fracture. The use of bisphosphonates for 5 years or longer, compared with transient or no use, was associated with a significantly increased risk of atypical hip or femur fracture (odds ratio [OR] = 2.74; 95% CI, 1.25-6.02). Among the 52,595 women using bisphosphonates for at least 5 years, a subtrochanteric or femoral shaft fracture occurred in 188 (0.36%) within 2 years. Duration of therapy of less than 5 years was not associated with an increased risk of fracture.
Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA. 2011;305(8):783-789.
- What Will Be the Future for Long Time Fosamax Users? – While jumping rope with the neighborhood children, a 59 year old Queens, New York woman felt her thigh bone snap. The pain was so severe that she fell to the ground as she readied for another jump. Sandy Potter had been diagnosed at the age of 48 with osteoporosis and began taking the drug Fosamax. She further stated that she had been on the medication for eight years prior to the incident and was now informed that her femur had snapped into two separate pieces.
-
Revisiting Bisphosphonates and Femur Fractures
 I have written a number of pieces about the problems associated with bisphosphonates and osteonecrosis of the jaw. Now, there is this piece about some additional problems with these drugs.
I have written a number of pieces about the problems associated with bisphosphonates and osteonecrosis of the jaw. Now, there is this piece about some additional problems with these drugs.Nearly six years ago in this column, I discussed what was then a little-known problem associated with long-term use of bisphosphonates, the valuable drugs that protect against fractures caused by bone loss. The drugs, among them Fosamax, Actonel and Boniva, can slow bone loss, increase bone density and cut fracture rates in half in women with established osteoporosis.
Reports had begun to emerge that some women taking bisphosphonates for many years suffered an unusual fracture of the femur, the long bone of the thigh. There was little or no trauma; in most cases the women were simply standing or walking when the femur snapped in half. In some, breaks occurred in both thighs, and many of the fractures were unusually slow to heal.
Experts think the fractures happened because of the way the drugs work: by slowing the rate of bone remodeling, the normal process by which injured bone heals. As a result, microfractures that occur through normal wear and tear are not repaired. Although bone density may be normal, the bone can become brittle and crack under minor stress.
In the years since, hundreds of cases of atypical femur fractures have been reported among women and some men taking bisphosphonates for five or more years. A number of studies have tried to assess the risk, and last fall the Food and Drug Administration issued a “safety announcement” and required that the drugs’ labels warn physicians and patients to be alert for this potential complication.
So, with all of the problems associated with these drugs and the realized benefit, do the risks outweigh the benefits? The latest study was published in JAMA on February 23rd.
“Compared to the number of fractures prevented,” she said, “the actual risk of a subtrochanteric femur fracture is small” — 1 case in 1,000 in the sixth year of therapy and 2.2 cases in 1,000 the seventh year.
And, in another report in the The New England Journal of Medicine.
A report published last year in The New England Journal of Medicine found no increase in atypical femur fractures, but that study did not include enough patients taking bisphosphonates for many years to produce a reliable result. Preliminary data from a much larger study has indicated that the risk of atypical femur fractures increased from 2 cases a year per 100,000 users after two years of bisphosphonate therapy to 78 cases a year per 100,000 after eight years on the drug.
In a report from a 27-member task force of the American Society for Bone and Mineral Research (published online in September in The Journal of Bone and Mineral Research), the experts noted that the way bisphosphonates work can reduce the “toughness” of bones. “It is highly likely that case reports and case series of atypical femur fractures will continue to accumulate,” the task force wrote, noting that another 47 cases had been reported since their analysis was prepared. Many cases are not reported, and in an unknown number of cases physicians may not recognize the fractures as atypical.
The task force called for an international registry of cases, including details that could help define who is most at risk.
So, what should a patient and a dentist do?
Certainly, be aware of the inherent risks of the bisphosphonates and be sensitive to the need, particularly if you are not at a high fracture risk.
Initial excitement about bone-protecting drugs led to prescriptions for millions of women who were not necessarily at high fracture risk, and many experts now urge a thorough evaluation before a bisphosphonate is prescribed. In addition to bone density test results, the evaluation should take into account a patient’s smoking and drinking habits, thinness, family history of osteoporosis, previous osteoporotic fractures, drug prescriptions and weight-bearing exercise regimen. An online evaluation tool developed by the World Health Organization is at www.shef.ac.uk/FRAX, though some experts have criticized it as incomplete.
The task force said a decision to treat should be “based on an assessment of benefits and risks,” and added, “patients who are deemed to be at low risk of osteoporotic-related fractures should not be started on bisphosphonates.”
Even those with osteoporosis in the spine but little or no problem in their hips, the experts concluded, should consider alternative remedies.
Osteonecrosis of the jaw is NOT a minor complication and the dentist in consult with the patient and patient’s physician must evaluate the risks, prior to dental surgery.
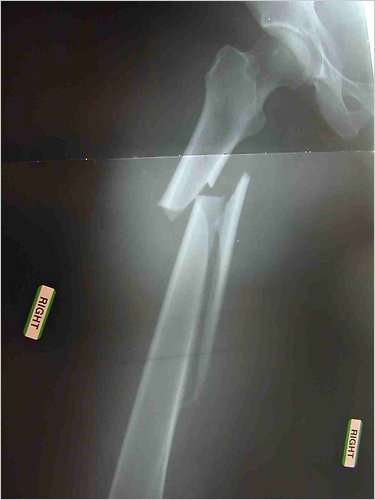
The femur of Dr. Jennifer Schneider of Tucson, an internist who after seven years on Fosamax suffered a nontraumatic femur fracture that took two years to heal
Dr. Schneider invites patients who have had such a fracture to write her at jennifer@jenniferschneider.com
Previous:
New Dentistry Cause for Alarm for Patients Who Use Bisphosphonates – Fosamax, Actonel, Boniva?
-
New Dentistry Cause for Alarm for Patients Who Use Bisphosphonates – Fosamax, Actonel, Boniva?
Cross Posted from Flap’s Dentistry BlogA new study coming out of Flap’s alma mater, the University of Southern California School of Dentistry has reported an increased incidence in the occurrence of osteonecrosis of the jaw among patients taking oral bisphosphonates, including Fosamax, Actonel and Boniva.In a separate report Thursday in the Journal of the American Dental Assn., Parish Sedghizadeh of the USC School of Dentistry reported that he had observed nine cases of osteonecrosis of the jaw among 208 patients taking oral bisphosphonates — an unusually high incidence.
Osteonecrosis of the jaw — death of the bone — is known to occur in 1% to 10% of cancer patients taking intravenous bisphosphonates to combat bone weakening brought on by chemotherapy. It is rarely observed in patients taking oral forms of the drug, however.
Some people immediately dismissed his findings, arguing that the widespread use of the drugs would have already revealed such a high incidence of a disabling side effect.
“The most important criticism is that the sample size was only 208 patients,” said Dr. Robert R. Recker, director of the Osteoporosis Research Center at Creighton University in Omaha and president of the National Osteoporosis Foundation. “The study design and sample size were simply inadequate to make any conclusions.”
Both the American Society for Bone and Mineral Research and the American Dental Assn. have recently conducted comprehensive reviews of the risks of osteonecrosis associated with oral bisphosphonates, added Dennis Black, a professor of epidemiology and biostatistics at UC San Francisco. They concluded “that the risk is somewhere between 1 in 10,000 and 1 in 100,000, and it is not clear that this is increased by bisphosphonates,” he said.
The American Dental Association today issued the following advisory which is here.
Most noteworthy from the advisory:
- The American Dental Association has reviewed previous studies on this issue which indicate that use of oral bisphosphonates (medication commonly prescribed for patients with osteoporosis) has been associated with a low risk for developing ONJ-one study indicated 1 out of 2,260 people taking oral bisphosphonates develop ONJ. This new study from USC claims that the frequency of ONJ among oral bisphosphate users is higher than previously reported-approximately 4 percent of the people studied.
- It’s important for me to know if you are on bisphosphonate drugs or if you’ve used them in the past because of the possible risk of developing ONJ. ONJ is uncommon, but it can be serious. If you have received bisphosphonate therapy intravenously related to cancer therapy, you may be at a higher risk of developing ONJ than if you take oral medication.
- Current ADA recommendations suggest that routine dental treatment generally need not be altered merely because a patient is taking or has taken oral bisphosphonates. However, patients with any history of bisphosphonate therapy should be especially encouraged to practice optimal oral hygiene and to receive routine dental examinations. Furthermore, a comprehensive oral examination and treatment before or soon after commencing bisphosphonates therapy may be beneficial for patients if they’re not already receiving regular dental care to treat existing oral health problems
A previously issued patient handout is available for download here (pdf).
This is a controversy which will be further studied.
In the meantime, Flap urges caution for patients taking ORAL Bisphosphonate medications. And,please patients update your health history and tell your dentist if you are using these drugs.
Previous:
Technorati Tags: Bisphosphonate, Fosamax, Actonel, Boniva, Osteonecrosis of the Jaw