-
FDA Panel Says Bisphosphonates Need Label Changes on Use Duration

This is the recommendation that came down today although the Food and Drug Administration does not have to accept the recommendations of its panels.Bone drugs from Warner Chilcott Plc, Roche Holding AG, Merck & Co. and Novartis AG need labeling changes to reduce the risk of fractures, a U.S. panel said.
The companies should add clarifications on the length of time that osteoporosis patients may take the medicines, outside advisers to the Food and Drug Administration said today in a 17- 6 vote in Adelphi, Maryland. The FDA isn’t required to follow its panels’ recommendations.
The agency has evaluated the safety of the drugs, known as bisphosphonates, for almost four years and cited possible links to unusual thigh fractures and jawbone deterioration in 2010. The agency said in July it also was examining conflicting studies on whether bisphosphonate pills such as Warner Chilcott’s Actonel, Merck’s Fosamax and Roche’s Boniva raise esophageal cancer risks.
A revised label should “be very clear that efficacy may fall off after a period of time, perhaps five years,” panelist Lewis Nelson, director of the medical toxicology fellowship program at New York University, said after the vote. “Serious concerns have been raised about risk, and those need to be continually evaluated as well.”
There needs to be additional research, period.
Mere warning labels are not going to answer the questions from every day patients – how long do I take the medicine and what protocol do I use? Or, what is the chance my femur will fracture or will I develop osteonecrosis of the jaw (ONJ) if I have a tooth removed.
-
Fosamax Osteonecrosis of the Jaw Lawsuit Set for Trial September 7

Osteonecrosis of the Jaw (ONJ)
Another oral bisphosphonate (Fosamax with ONJ) lawsuit trial set.The next trial date for a Fosamax lawsuit involving jaw necrosis is scheduled to begin early next month.
The Fosamax trial will involve a complaint brought by Linda Secrest, of Florida, who alleges that Merck failed to adequately warn that side effects of Fosamax, the popular osteoporosis drug, can lead to severe jaw bone decay. Trial is scheduled to begin on September 7, in the U.S. District Court for the Southern District of New York.
Fosamax is an osteoporosis drug that belongs to a family of similar medications known as bisphosphonates. Long-term use of oral bisphosphonates has been linked to an increased risk of serious and debilitating jaw problems, known as osteonecrosis of the jaw. The condition causes the jaw bone to decay and rot, often resulting in the need for surgery to remove portions of the jaw.
Merck currently faces more than 1,100 Fosamax jaw lawsuits, most of which have been consolidated and centralized for pretrial proceedings in the U.S. District Court for the Southern District of New York as part of an MDL or multidistrict litigation.
There have already been several trials for Fosamax jaw necrosis lawsuits that have been held in the federal MDL over the last two years. While Merck has successfully defended its medication in three cases, one lawsuit resulted in an $8 million jury award for Fosamax jaw damage last year, finding that Merck failed to adequately research the potential side effects of warn about the risk of jaw necrosis from Fosamax.
A few more cases and Merck will probably propose some industry-wide settlement agreement.
Next up will be the lawsuits over bone fractures from Fosamax.
-
Oral Bisphosphonates: Study – Absolute Risk for Femur Fracture Low with Bisphosphonates
 I have written a number of pieces about the problems associated with bisphosphonates and osteonecrosis of the jaw. My latest post is here.
I have written a number of pieces about the problems associated with bisphosphonates and osteonecrosis of the jaw. My latest post is here.Now, there is a new study published in the New England Journal of Medicine which sheds new light on the advantages vs. risks of taking these drugs.
Almost 78% of all Swedish women aged 55 years and older who sustained an atypical femur fracture in 2008 had taken bisphosphonates, but the absolute risk for such breaks is small enough to justify prescribing the drugs, according to a study published in the May 5 issue of the New England Journal of Medicine (NEJM).
For patients taking bisphosphonates, the age-adjusted relative risk for an atypical femur fracture — a clean, horizontal break spreading from the lateral side and occurring with minimal or no trauma — was 47.3%, the study reported. The crude incidence of the fractures was 0.09 per 10,000 patient-years among women who had never taken the drug compared with 5.5 among those who had ever taken it.
These results “should be reassuring for bisphosphonate users,” write lead author Jörg Schilcher, MD, from the Department of Experimental and Clinical Medicine, Faculty of Health Science, Linköping University, Linköping, Sweden, and colleagues. “With a correct indication, the benefits of fracture prevention…will greatly outweigh the risk of atypical femoral fracture.”
So, in other words, the benefits of preventing fractures by taking the medications outweigh the risk of an atypical leg (femur) fracture.
So, how does this apply to dentistry?
With more and more patients taking these drugs to prevent osteoporosis, and for longer periods of time, dentists will have to be scrupulous in their medical histories. Osteonecrosis of the jaw, while rare, is a known and serious complication.
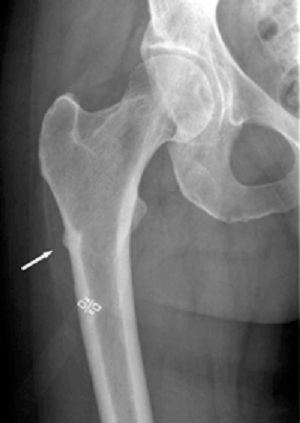
An undisplaced femoral fatigue fracture associated with bishosphonate treatment. NIH photo
This study echoes the paper published in February in the Journal of the American Medical Association which I cited a few months ago. The AMA paper is here.Experts interviewed by Medscape Medical News call the study definitive because researchers not only studied a massive number of participants — all 1.5 million women in Sweden who were aged 55 years or older in 2008 — but also reviewed the x-rays of nearly all those who had particular kinds of femur fractures.
“It’s the largest and most comprehensive study of this issue that I’ve seen,” said Sundeep Khosla, MD, president of the American Society for Bone and Mineral Research (ASBMR) and a professor of medicine and physiology at the Mayo Clinic in Rochester, Minnesota.
The study’s conclusions echo those in other recent studies on the worrisome fractures and the bone-building drugs for osteoporosis. An article published in February in the Journal of the American Medical Association, for example, reported that long-term use of bisphosphonates boosted the risk for these fractures, but those authors noted that the absolute risk for fracture is low and is outweighed by the benefits of the therapy.
And, what about drug holidays – especially since the risk of fracture declines rapidly after drug withdrawal?
Because it is not clear how long patients with osteoporosis can be safely treated with bisphosphonates, the ASBMR recommends that clinicians consider discontinuing them after 5 years. At that point, many physicians give their patients a “drug holiday” for 1 or 2 years and then resume the therapy. Dr. Shane said that the rapid decline in fracture risk after drug withdrawal helps justify a holiday.
“Now there seems to be evidence that giving patients intermittent drug holidays is appropriate to do,” she said. Dr. Khosla agreed, saying the decrease in fracture risk provides “reassurance that a [drug holiday] is good clinical practice.”
Again for dentists, it will be esepcially important to monitor our patients for when they are on or off the medications. Since dental visits may be episodic, and in older patients only in case of an emergency e,g. tooth extraction, proper dental/medical records are mandatory. Appropriate consultation with the patient’s physician is indicated. Treatment plans for dental examination and/or treatment either prior to initial treatment or before resumption of treatment with these drugs would be beneficial to patients.
I will look forward to additional studies on the risk of dental complications using a drug holiday approach – with resumption of drug use.
In the meantime, Flap urges caution for patients taking ORAL Bisphosphonate medications. And,please patients update your health history and tell your dentist if you are using these drugs.
Previous:
Revisiting Bisphosphonates and Femur FracturesNew Dentistry Cause for Alarm for Patients Who Use Bisphosphonates – Fosamax, Actonel, Boniva?
-
Revisiting Bisphosphonates and Femur Fractures
 I have written a number of pieces about the problems associated with bisphosphonates and osteonecrosis of the jaw. Now, there is this piece about some additional problems with these drugs.
I have written a number of pieces about the problems associated with bisphosphonates and osteonecrosis of the jaw. Now, there is this piece about some additional problems with these drugs.Nearly six years ago in this column, I discussed what was then a little-known problem associated with long-term use of bisphosphonates, the valuable drugs that protect against fractures caused by bone loss. The drugs, among them Fosamax, Actonel and Boniva, can slow bone loss, increase bone density and cut fracture rates in half in women with established osteoporosis.
Reports had begun to emerge that some women taking bisphosphonates for many years suffered an unusual fracture of the femur, the long bone of the thigh. There was little or no trauma; in most cases the women were simply standing or walking when the femur snapped in half. In some, breaks occurred in both thighs, and many of the fractures were unusually slow to heal.
Experts think the fractures happened because of the way the drugs work: by slowing the rate of bone remodeling, the normal process by which injured bone heals. As a result, microfractures that occur through normal wear and tear are not repaired. Although bone density may be normal, the bone can become brittle and crack under minor stress.
In the years since, hundreds of cases of atypical femur fractures have been reported among women and some men taking bisphosphonates for five or more years. A number of studies have tried to assess the risk, and last fall the Food and Drug Administration issued a “safety announcement” and required that the drugs’ labels warn physicians and patients to be alert for this potential complication.
So, with all of the problems associated with these drugs and the realized benefit, do the risks outweigh the benefits? The latest study was published in JAMA on February 23rd.
“Compared to the number of fractures prevented,” she said, “the actual risk of a subtrochanteric femur fracture is small” — 1 case in 1,000 in the sixth year of therapy and 2.2 cases in 1,000 the seventh year.
And, in another report in the The New England Journal of Medicine.
A report published last year in The New England Journal of Medicine found no increase in atypical femur fractures, but that study did not include enough patients taking bisphosphonates for many years to produce a reliable result. Preliminary data from a much larger study has indicated that the risk of atypical femur fractures increased from 2 cases a year per 100,000 users after two years of bisphosphonate therapy to 78 cases a year per 100,000 after eight years on the drug.
In a report from a 27-member task force of the American Society for Bone and Mineral Research (published online in September in The Journal of Bone and Mineral Research), the experts noted that the way bisphosphonates work can reduce the “toughness” of bones. “It is highly likely that case reports and case series of atypical femur fractures will continue to accumulate,” the task force wrote, noting that another 47 cases had been reported since their analysis was prepared. Many cases are not reported, and in an unknown number of cases physicians may not recognize the fractures as atypical.
The task force called for an international registry of cases, including details that could help define who is most at risk.
So, what should a patient and a dentist do?
Certainly, be aware of the inherent risks of the bisphosphonates and be sensitive to the need, particularly if you are not at a high fracture risk.
Initial excitement about bone-protecting drugs led to prescriptions for millions of women who were not necessarily at high fracture risk, and many experts now urge a thorough evaluation before a bisphosphonate is prescribed. In addition to bone density test results, the evaluation should take into account a patient’s smoking and drinking habits, thinness, family history of osteoporosis, previous osteoporotic fractures, drug prescriptions and weight-bearing exercise regimen. An online evaluation tool developed by the World Health Organization is at www.shef.ac.uk/FRAX, though some experts have criticized it as incomplete.
The task force said a decision to treat should be “based on an assessment of benefits and risks,” and added, “patients who are deemed to be at low risk of osteoporotic-related fractures should not be started on bisphosphonates.”
Even those with osteoporosis in the spine but little or no problem in their hips, the experts concluded, should consider alternative remedies.
Osteonecrosis of the jaw is NOT a minor complication and the dentist in consult with the patient and patient’s physician must evaluate the risks, prior to dental surgery.
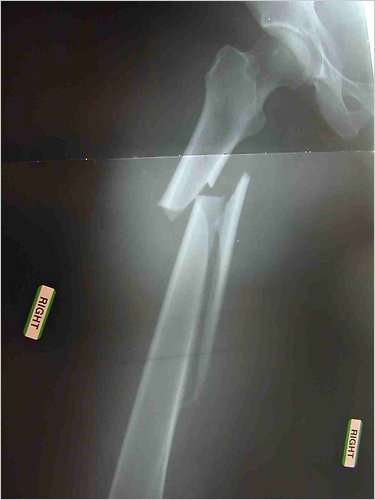
The femur of Dr. Jennifer Schneider of Tucson, an internist who after seven years on Fosamax suffered a nontraumatic femur fracture that took two years to heal
Dr. Schneider invites patients who have had such a fracture to write her at jennifer@jenniferschneider.com
Previous:
New Dentistry Cause for Alarm for Patients Who Use Bisphosphonates – Fosamax, Actonel, Boniva?